Head Diagnostics and RCSI Announce Clinical Study Partnership to Improve Assessment and Monitoring of MS
Head Diagnostics, headquartered at NovaUCD in Dublin, is partnering with RCSI on a clinical study to enhance the assessment and monitoring of multiple sclerosis (MS) through innovative technology. The study, launched to coincide with World MS Day, will explore the use of novel digital biomarkers to monitor disease, with the aim of providing greater objectivity in assessing the condition.
MS is a complex neurological disorder that affects millions of people worldwide. The current methods of assessing disease progression and treatment effect for MS patients are limited and often subjective. This new collaboration aims to address this issue by exploring the potential of non-invasive digital biomarkers, including a little-known tremor of the eye and measures of a person’s manner of walking, to provide accurate and real-time data on disease activity.
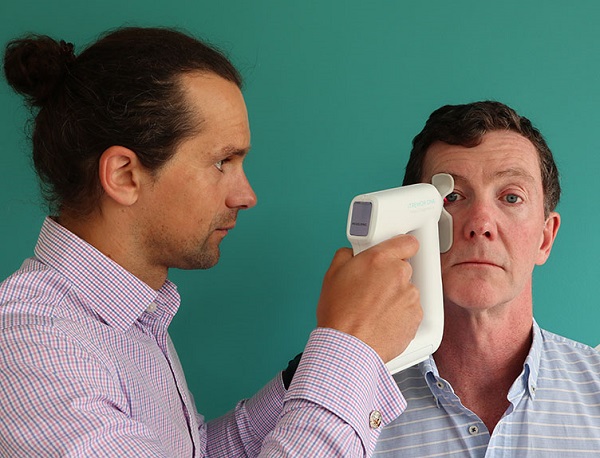
Central to the study is Head Diagnostics' innovative iTremor One device, a handheld medical device that gives a rapid and non-invasive measure of ocular microtremor (OMT), a high-frequency involuntary fixational eye movement that is believed to reflect activity in the brainstem. Previous studies have found that OMT changes in people with MS, as well as other neurological conditions, although the traditional approach to measuring this has been particularly invasive, limiting research.
Combining this measure with gait analysis offers the potential to provide more comprehensive, accurate and real-time data on disease activity.
This observational study based in Beaumont Hospital is designed to evaluate the utility of OMT and gait in monitoring MS, enrolling 120 participants including those with and without a diagnosis of MS, and assessing them regularly over a 12-month period.
“Exploring the potential of measuring patients’ microtremors of the eye and their gait as measures of MS progression offers a promising new direction in our management approach,” said Dr Lisa Costelloe, Honorary Clinical Senior Lecturer at RCSI, Consultant Neurologist at Beaumont Hospital, and study co-principal investigator.
“The treatment options for MS patients have advanced dramatically in recent decades but the outcome measures we use in clinical practice do not capture all aspects of the disease, in particular early disease progression. This study marks a significant step in exploration of more precise and robust monitoring of MS, which is crucial for effective management and treatment.”
Dr Rob Argent, Lecturer in Digital and Connected Health in RCSI’s School of Pharmacy and Biomolecular Sciences and study co-principal investigator, said, “This study presents a unique opportunity to develop digital measures that can provide a more convenient, comprehensive, and objective measure of MS. By leveraging digital health technologies, we seek to support assessment, enhance disease monitoring, and contribute to the advancement of precision medicine in MS.”
“In combining our innovative iTremor One device with RCSI's research expertise, we aim to revolutionise the way MS is assessed and monitored. This study supports our mission to provide objective and real-time disease management tools for neurological diseases and impairments,” said, David van Zuydam, CEO, Head Diagnostics.
This study is supported by the Enterprise Ireland Innovation Partnership Programme, which provides funding and support for collaborative research projects between industry and academic institutions in Ireland.
ENDS
30 May 2024
For further information contact Micéal Whelan, Communications and Media Relations Manager, NovaUCD, t: + 353 1 716 3712, e: miceal.whelan@ucd.ie.
Editors Notes
For further information about Head Diagnostics visit: https://headdiagnostics.com/
For further information about RCSI visit: https://www.rcsi.com/
