A new blood test for inflammation that helps to identify patients who may be at increased risk of future stroke and heart disease
Monday, 25 November, 2024
Share

Congratulations to UCD School of Medicine's Dr John McCabe, Dr Sarah Gorey, Professor Peter Kelly, and all the team involved on their recently published research in the American Heart Association Journal, Stroke, titled, Interleukin-6, C-Reactive Protein, and Recurrence After Stroke: A Time-Course Analysis of Individual-Participant Data. The paper has been published in print today, 25 November 2024.
The research includes contributors from 11 prospective studies and 10,000 patients.
Stroke is a leading cause of disability and death, with over 100 million people living life after stroke globally. There is an urgent need for new treatments to prevent stroke and to develop new blood tests that will identify who is at greatest risk. In collaboration with 10 academic partners around the world, researchers at UCD have led out on a project involving 10,000 stroke patients to identify a new blood test for inflammation that helps to identify patients who may be at increased risk of future stroke and heart disease. The research was led by Dr John McCabe, Professor Peter Kelly, and Professor Cathal Walsh.
Dr John McCabe stated, "We hope that our work will pave the way to develop new stroke prevention treatments that lower inflammation in patients after stroke and provide new therapies that are desperately needed."
Abstract
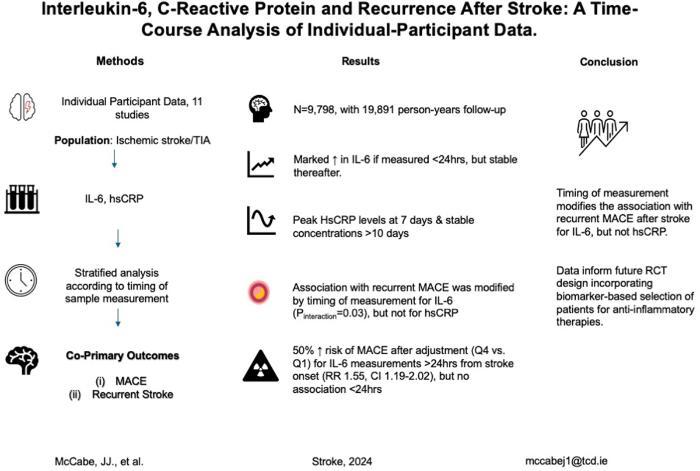 Inflammation promotes atherogenesis. Randomized controlled trials of anti-inflammatory therapies for prevention after stroke have not yet demonstrated clear benefit. IL-6 (interleukin-6) and hsCRP (high-sensitivity C-reactive protein) are independently associated with major adverse cardiovascular events poststroke and may guide patient selection in future randomized controlled trials. Optimal timing of hsCRP/IL-6 measurement poststroke is unknown, as early blood levels may be confounded by the inflammatory response to brain infarction.
Inflammation promotes atherogenesis. Randomized controlled trials of anti-inflammatory therapies for prevention after stroke have not yet demonstrated clear benefit. IL-6 (interleukin-6) and hsCRP (high-sensitivity C-reactive protein) are independently associated with major adverse cardiovascular events poststroke and may guide patient selection in future randomized controlled trials. Optimal timing of hsCRP/IL-6 measurement poststroke is unknown, as early blood levels may be confounded by the inflammatory response to brain infarction.
METHODS:
RESULTS:
CONCLUSIONS: