Translational Research - Drug Delivery Group
Research in the UCD School of Veterinary Medicine from the Lab of Professor David Brayden
David Brayden is Professor of Advanced Drug Delivery at UCD. He is a pharmacologist and joined UCD in 2001 following 10 years in the pharmaceutical industry at Elan Biotechnology Research working on drug delivery research. He is a Fellow of the American Association of Pharmaceutical Scientists and of the Controlled Release Society. In the intervening years, his research at the UCD School of Veterinary Medicine has spanned a number of areas of drug transport, most with application in both human and veterinary medicine and in keeping with the 'One Health' theme.
Grants supporting his PhD candidates and Postdoctoral Fellows have been from Science Foundation Ireland, the Irish Research Council, the Health Research Board, the EU FP and Horizon programmes, and from the Pharma industry. He has been continuously funded by SFI since 2005 from his initial Investigator Grant through to Directing the Irish Drug Delivery Research Network Cluster from 2007-2012, and he is a current Co-Lead Principal Investigator of the SFI CURAM Centre for Medical Devices (2012- ). Graduated PhDs from the School that he has supervised include: Simon Keely (2006), Linda Feighery (2006), Jyoti Soni (2007), Xuexuan Wang (2009), Lee-Ann Rawlinson (2010), Victoria Bzik (2011). Ed Walsh (2013), Tanira Aguirre (2013), Tauseef Ahmad (2015), Fiona McCartney (2016), John Gleeson (2017), and Svenja Sladek (2017). Caroline Twarog, Vivien Stuettgen, and Joanna Heade will submit their theses in 2020. Postdoctoral Fellows include Joanna Griffin (2008), Sinead Ryan (2008), Signe Petersen (2012), Fiona McCartney (2016), Delyan Hristov (2016), Jason Beirne (2016), Sarinj Fattah (2017), Rumi Khandelia (2019-), and Mustafa Abeer (2019-) Most of these researchers are now employed in the Pharmaceutical industry or in academia.
In 2005, one of the lab’s highlights was on a veterinary project funded by Pfizer Animal Health (now Zoetis). Pfizer wanted to know if their anti-parasitic product, selamectin, was subject to efflux from the gut by a transporter, P-glycoprotein (P-gp). This was important because it was known that the main drug of the class, ivermectin, was subject to efflux in the gut and blood-brain barrier, and the question was whether, like ivermectin, selamectin could be neurotoxic in dogs with a mutation in P-gp. Using transfected cell lines for P-gp and other efflux pumps, a postdoc in Brayden’s group, Joanna Griffin, proved that selamectin was indeed a substrate and inhibitor of P-gp. “Selamectin is a potent substrate and inhibitor of human and canine P-glycoprotein” by Griffin J, Fletcher N, et al, was published in the Journal of Veterinary and Pharmacological Therapeutics and won the journal’s Best Paper award that year. One of the paper’s co-authors, Nicola Fletcher (DVM, PhD), will join the School as an Ad Astra Assistant Professor in 2020. So, why doesn’t selamectin pose the same neurotoxic problems as ivermectin? The answer was that selemectin was formulated only as a topical oily agent for cats and dogs and therefore could not be abused to dose dogs orally in excessively high doses by some farmers, who had previously leveraged the unapproved cattle formulation of ivermectin for their dogs - with disastrous results for P-gp mutated dogs. 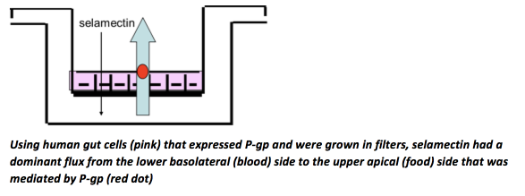
More recently, the lab has been researching how peptides like insulin can be incorporated in nanoparticles for oral delivery in preference to injections. Typically, such molecules breakdown in the gut and cannot cross the wall as they are too big and too soluble. This is why diabetic humans and small animals require insulin injections. A recent discovery by the group was that insulin could be entrapped in a silica-based nanoparticle and it had the capacity to reduce blood glucose in a rat model. Postdocs Fiona McCartney and Delyan Hristov found that it was an innocuous excipient or formulation component that caused this benefit to occur: the particle released the insulin close to the gut wall and the excipient then helped it across the wall to the blood vessel that led to the liver. The discovery, which was the subject of a patent application, challenged the literature, which had been emphasising that the success of similar particles in oral delivery was solely due to particle uptake by the gut wall. This has potential application for development of oral tablets of insulin and other peptides for human and animal patients. The lab’s work on oral insulin was recently highlighted in the RTE documentary, “Bittersweet,” which won the “Raw Science” Documentary Prize in Los Angeles in 2018. 
A third example also relates to a nanoparticle, but this time as a potential treatment for inflammatory arthritis. The team had unfortunately failed with a nanoparticle comprising the peptide, calcitonin, designed for oral delivery as a treatment for osteoporosis - because not enough calcitonin was absorbed. So they hit on the idea that calcitonin might work as an anti-inflammatory agent in arthritic joints if it could be presented in a nanoparticle formulated with a polymer and with the anti-inflammatory natural lubricant, hyaluronic acid sugar. When they injected the nanoparticle to the knee of a mouse model of arthritis, inflammation was partially resolved and the effect was on a par with steroids. Moreover, as the School has a real strength in inflammation biology, a collaboration with Professor Evelyn Murphy, helped work out the cellular basis of effect, a down-regulation of a transcription family of molecules associated with inflammation. This work was published in the Journal of Controlled Release in 2013: “An intra-articular salmon calcitonin-based nanocomplex reduces experimental inflammatory arthritis” by Ryan SM et al. Lead author, Sinead Ryan, subsequently became an Assistant Professor in the School in 2014. The group took this work further. Taking advantage of being in a Vet School, they had potential access to horses that are used for research and teaching at the UCD Lyons Farm to test the nanoparticles. Working with Prof Pieter Brama, an expert in equine osteoarthritis, they designed an ethically-approved HPRA-licenced study to test the particles as a knee injection in this large animal model. The team had to work out the translating dose from mouse to horse, and then had to ensure that a scaled-up sterile product could be produced. This was achieved using facilities at the National Institute of Bioprocessing and Training (NIBRT). The result showed that the nanoparticles were safe in the horses and that they reduced expression of a number of inflammatory biomarkers. This work was published in Drug Delivery and Translational Research in 2018 and was the basis of Svenja Sladek’s Ph.D. 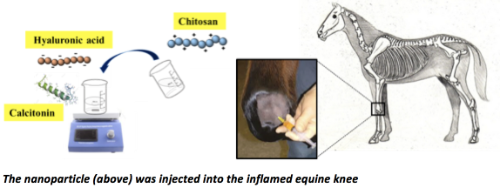
New work in the lab is focussing on how peptides with beneficial properties isolated from food can be absorbed by the gut. This project is funded by Nuritas Ltd and involves working out which of their proprietary peptides are permeable enough across the gut wall so that they can be synthesised into oral tablet formulations. There is also an EU Marie Curie project led by Rumi Khandelia, in which an anti-inflammatory nanoparticle made from albumin protein and an established anti-inflammatory molecule has been designed for joint injection for arthritis.